Amp up your revenue pipeline automatically.
Get the programs and prospects that precisely match your company’s capabilities—in a flash. Know when your targets are ready to engage and be armed with insights to wow the dealmakers. Amplion is purpose-built for commercial teams selling to pharma and academia and delivers results while you sleep.
JOIN LEADING LIFE SCIENCE COMPANIES

“After a few months of implementing Amplion, we’re now setting records in terms of our monthly lead generation activities.”
Paul DiGregorio
VP Commercial, NanoCellect
How does Amplion Work for Me?

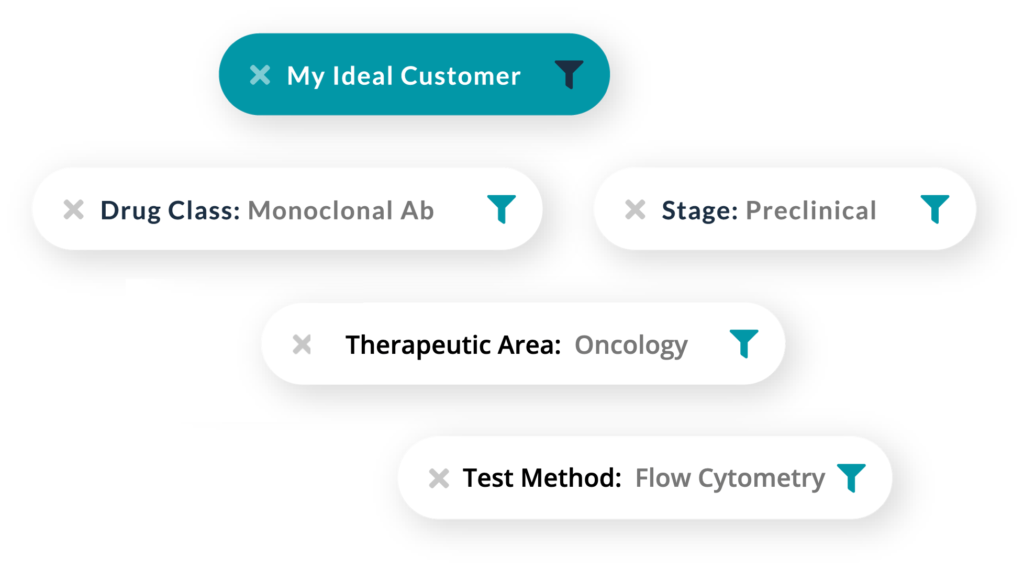
Shrink the haystack to reveal your ideal customers
Amplion analyzes millions of pieces of biomedical and business evidence, while enabling you to define detailed criteria for leads that exactly match your capabilities. Your targeted opportunities will be waiting for you on day one with tailored, pre-set parameters that put you in your signal-to-noise sweet spot.
Stay on top of your best opportunities
Flag organizations and programs for immediate access anytime, then you’ll get automatic alerts for meaningful happenings as they unfold. Favorite and tag the most intriguing, actionable events so you can streamline your workflow and ditch the spreadsheets.

Monitor critical sales signals while you sleep
Reclaim your schedule with real-time push notifications of events and new leads, so you can track all the activities that are relevant to your business—effortlessly. Insights delivered to your inbox let you reimagine what you can accomplish every day.
Discover real, relevant contacts
Amplion sources contacts from publications, clinical trial records and public databases to help you identify key decision makers at your target organizations. Continuous updates mean you get the most current contact info to ensure your success.

![]() SALES
SALES
Rev up your sales engine
Discover more of your ideal prospects
Match to hundreds of organizations, programs and key contacts that fit your ideal profile, with sales signals delivered automatically, to help your team amp up productivity and identify opportunities before your competitors.
![]() MARKETING
MARKETING
Make your campaigns go further, faster
Innovate your marketing programs with complete data
Real-time insights let you craft messages that resonate to capture more leads, enhance lead scoring to accelerate your funnel velocity, and execute targeted campaigns with the right audience at exactly the right time.
![]() BUSINESS DEVELOPMENT
BUSINESS DEVELOPMENT
Unearth targeted partnership opportunities
Know where your best deals are right now
Real-time, deep account insights tailored to you and delivered automatically help you find the most lucrative deals faster, with so much less effort than ever before.
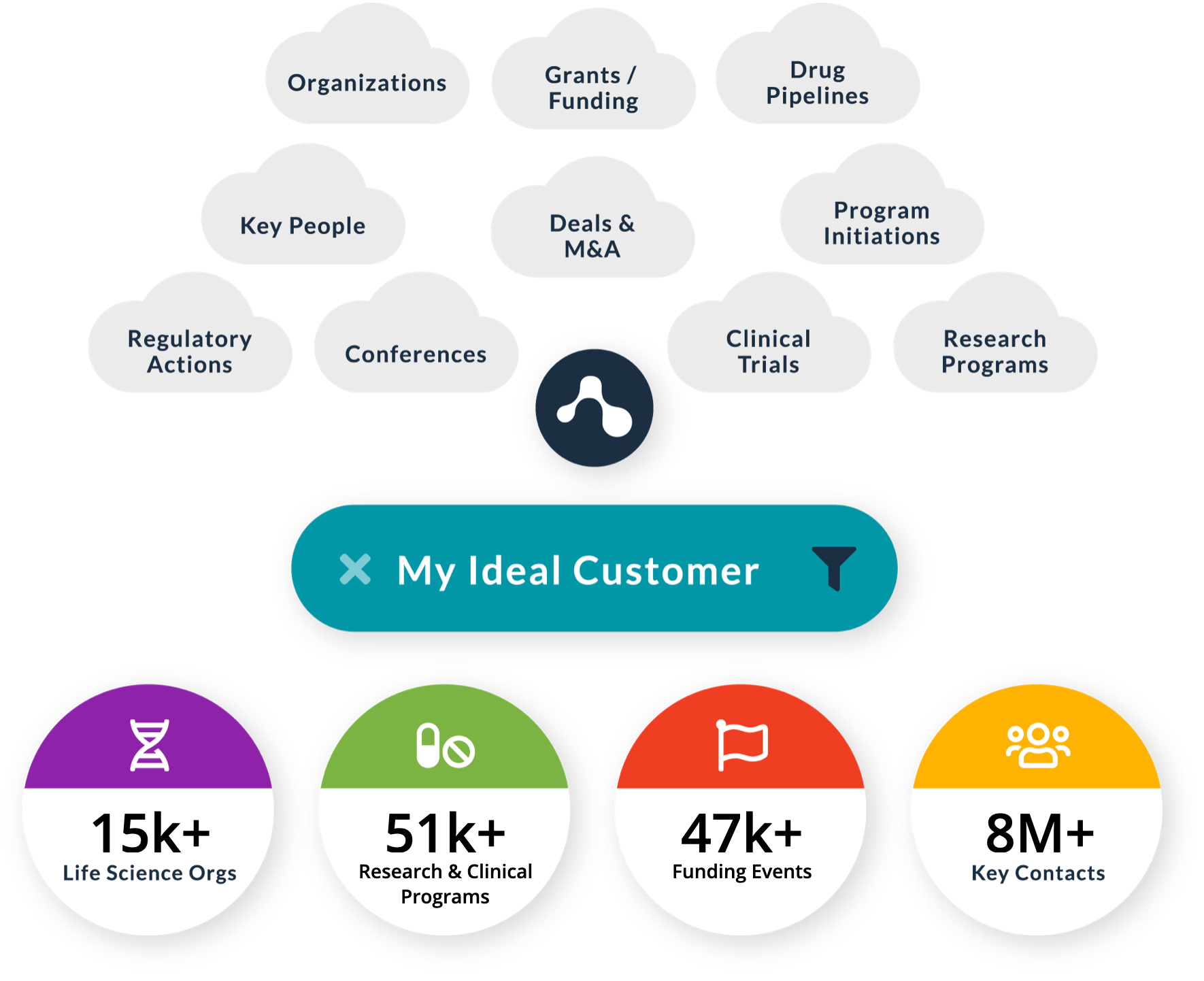
Chart your course with deep data
Over 33 million public and private data sources, combined with unmatched, proprietary machine learning make Amplion the powerhouse you’ve always needed. It finds everything you might otherwise miss and works 24/7, so you don’t have to.
AMPLION INTEGRATIONS
Integrated intelligence that fits your workflow
All of your critical data sources in one place. Targeted accounts, relevant sales signals and key contacts – pushed to your CRM.

What makes Amplion different?
Amplion is purpose-built for life science commercial teams to enable partnerships and collaborations that advance health. We pair deep scientific and business data with novel machine learning to help you fast forward your results. And we dream big right alongside you, working together to help you amplify your effort and crush your goals.
AMP UP YOUR LEAD GEN
Schedule a demo today
Experience why industry leaders trust Amplion to drive their most critical commercial goals forward, risk-free!
